Genes encoding lipid transfer proteins (LTPs) are epigentic changed in peripheral blood mononuclear cells (PBMC) from ME patients (4, 5, 6, 7).
Protein levels of LTPs are changed in the spinal fluid from ME patients (8).
Lipid Transfer Proteins
Within the eukaryotic cell, more than 1000 species of lipids define a series of membranes essential for cell function. Tightly controlled systems of lipid transport underlie the proper spatiotemporal distribution of membrane lipids, the coordination of spatially separated lipidmetabolic pathways, and lipid signaling mediated by soluble proteins that may be localized some distance away from membranes. Alongside the well-established vesicular transport of lipids, non-vesicular transport mediated by a group of proteins referred to as lipid-transfer proteins(LTPs) is emerging as a key mechanism of lipid transport in a broad range of biological processes. More than a hundred LTPs exist in humans and these can be divided into at least ten protein families. LTPs are widely distributed in tissues, organelles and membrane contact sites (MCSs), as well as in the extracellular space. They all possess a soluble and globular domain that encapsulates a lipid monomer and they specifically bind and transport a wide range of lipids (9).Fig. 5. Lipid-transfer proteins have multiple modes of action (Figure from Chiappariono et al. ref 9)
a, LTPs can transfer lipids between cellular membranes and act as transporters. b, Some LTPs (chaperones) present lipids to an acceptor protein (e.g. enzymes, LTPs, transmembrane (TM) transporters or transcription factors). c, The LTD can be engaged in intramolecular interactions with other domains (illustrated here in purple) or proteins (not illustrated). Binding to the lipid cargo acts as a trigger that induces conformational changes and leads to the activation of signaling. This mechanism is sometimes coupled to the mechanisms described in a and b. LTPs have pleiotropic functions and can modulate lipid homeostasis, signaling and the structural organization of membranes.
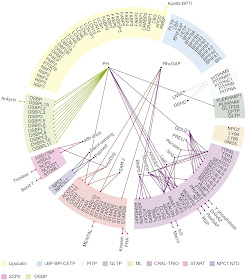
Fig. 4. Domain organizations of human lipid-transfer proteins. The members of the ten families of LTPs are displayed. (Figure from Chiapparino et al. ref 9).
PITPNA, PITPNC1 and PITPNM2 in ME
The genes encoding the phosphatidylinositol transfer proteins PITPNA, PITPNC1 and PITPNM2 were hypomethylated in PBMC from ME patients in Trivedi's study (7). PITPNM2 was hypomethylated in the genic regions: 1stExon, TSS200, TSS1500 and 3'UTR; and also showed changed DNA methylation pattern in 3 other studies (4, 5, 6).GM2A in ME
A number of LTPs can transfer lipids to downstream enzymes. GM2 ganglioside activator (GM2A) is a lysomal LTP that works as a cofactor for the glycosphingolipid-degrading enzyme beta-hexosaminidase A (HEXA). GM2A is also involved in presentation of antigenic lipids by CD1 to T cells.
The GM2A gene promoter was hypomethylated in ME patients (table S7 in ref 7).
GM2A precursor, number of unique peptides identified in cerebrospinal fluid (table S1 in ref 8):
1) Controls: 12
2) ME patients: 32
3) Post treatment Lyme patients:32
HEXA chain precursor, number of unique peptides identified in cerebrospinal fluid (table S1 in ref 8):
1) Controls: 16
2) ME patients: 30
3) Post treatment Lyme patients:28
PLTP in ME
The gene encoding phospholipid transfer protein (PLTP) was hypomethylated (TSS1500) in PBMC from ME patients (7).
PLTP (TSS1500) was differentially methylated in PBMC from ME patients subtypes (6).
PLTP (TSS1500) was differentially methylated in PBMC from ME patients subtypes (6).
PLTP precursor, number of unique peptides identified in cerebrospinal fluid (table S1 in ref 8):
1) Controls: 16
2) ME patients: 31
3) Post treatment Lyme patients: 26
STARD13 was differentially methylated in PBMC from ME patients subtypes (6).
This was some of the LTPs involved in ME.
STARD13 and ME
The gene encoding StAR related lipid transfer domain containing 13 (STARD13) was hypermethylated in genic region 3'UTR and hypomethylated in TSS200 in PBMC from ME patients (7).STARD13 was differentially methylated in PBMC from ME patients subtypes (6).
This was some of the LTPs involved in ME.
References
1) Naviaux RK, Naviaux JC, Li K, Bright AT, Alaynick WA, Wang L, Baxter A, Nathan N et al (2016) Metabolic features of chronic fatigue syndrome. Proc Natl Acad Sci U S A 113:E5472–E5480. https://doi.org/10.1073/pnas.16075711132) Germain et al: Metabolic profiling of a ME/CFS discovery cohort reveals disturbances in fatty acid and lipid metabolism. Mol. BioSyst. 2017, 13, 371 https://pubs.rsc.org/en/Content/ArticleLanding/2017/MB/C6MB00600K#!divAbstract
3) Nagy-Szakal et al: Insights into ME/CFS phenotypes through comprehensive metabolomics. Nat. Sci. Rep, 2018, 8. https://www.nature.com/articles/s41598-018-28477-9
4) de Vega et al: DNA methylation Modifications associated with CFS. PlosOne, 2014, 9, 8. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0104757
5) de Vega et al: Epigenetic modifications and glucocorticoid sensitivity in ME/CFS. BMC Medical Genomics, 2017, 10, 11 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5324230/
6) de Vega et al: Integration of DNA methylation & health scores identifies subtypes in ME/CFS. Epigenomics 2018, 10, 5 https://www.futuremedicine.com/doi/full/10.2217/epi-2017-015
7) Trivedi et al: Identification of ME/CFS - associated DNA methylation patterns.
Plos One 2018, 13, 7 https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0201066
8) Schutzer et al: Distinct Cerebrospinal Fluid Proteomes Differentiate Post- Treatment Lyme Disease from Chronic Fatigue Syndrome. PLOS One February 2011, volume 6, Issue https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0017287
9) Chiapparino et al: The orchestra of lipid-transfer proteins at the crossroads between metabolism and signaling. Prog Lipid Res. 2016 Jan;61:30-9. doi: 10.1016/j.plipres.2015.10.004 https://www.ncbi.nlm.nih.gov/pubmed/26658141

Ingen kommentarer:
Send en kommentar
Bemærk! Kun medlemmer af denne blog kan sende kommentarer.