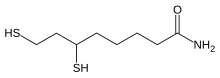
For at forstå hypotesen til bunds er det nødvendigt med basisviden om lipoic acid. Jeg vil derfor gennemgå basisviden fra wikipedia og fra artiklen:
Lipoic acid metabolism and mitochondrial redox regulation (2).
Jeg benytter de engelske ord for de kemiske forbindelser, så de er lettere at genfinde i wikipedia og i artiklen.
Lipoic acid (LA) bliver dannet ud fra caprylic acid (= octanoic acid). Octa betyder otte. Dvs. LA dannes af en fedtsyre, der har en kulstof kæde-længde på otte kulstof atomer (C). Fedtsyrekæden har fået sat et svovl atom på kulstof atom nr. 6 og 8. De to svovl atomer er bundet sammen i en disulfid-binding (-S-S-).

Disulfid-bindingen i lipoic acid leverer reduktionspotentiale til de tre 2-keto-acid dehydrogenase komplekser:
- PDC, pyruvate dehydrogenase complex
- OGDC, 2-oxo-glutarate dehydrogenase comples (2-oxo-glutarate = alpha-ketoglutarate)
- BCKDC, branched-chain alpha-keto dehydrogenase complex
Disse enzym komplekser er ens opbygget af tre subunits:
- E1: decarboxylase
- E2: lipoyltransferase
- E3: dihydrolipoamide dehydrogense
PDC subunits:
E1:
PDHA1, pyruvate dehydrogenase E1 alpha 1 subunit
E2: DLAT, dihydrolipoamide acetyltransferase
E3.
DLD, dihydrolipoamide dehydrogenase
Herudover findes der et E3 bindings-protein (E3BP), som binder E2 og E3 sammen. E3BP kaldes også for PDC component X og kodes af genet
PDHX.
OGDC subunits:
E1:
OGDH, oxoglutarate dehydrogenase
E2:
DLST, dihydrolipoamide S-succinyltransferase
E3.
DLD, dihydrolipoamide dehydrogenase
BCKDC subunits:
E1:
BCKDHA, branched chain keto acid dehydrogenase E1 subunit alpha og
BCKDHB branched chain keto acid dehydrogenase E1 subunit beta
E2:
DBT, dihydrolipoamide branched chain transacylase E2
E3.
DLD, dihydrolipoamide dehydrogenase
Som man kan se, benytter de tre enzym komplekser sammen E3 subunit: DLD. Herudover findes der et glycine cleavage system (GCS), som også benytter DLD.
GCS består af:
AMT, aminomethyltransferase
GLDC, glycine decarboxylase
DLD, dihydrolipoamide dehydrogenase
GCSH, glycine cleavage system protein H
Der findes endvidere et enzym 2-oxoadipate dehydrogenase (2-OADH), som også kaldes dehydrogenase E1 and transketolase domain containing 1 ((DHTKD1). DHTKD1 decarboxylerer 2-oxoadipate til glutaryl-CoA, som sidste trin i nedbrydning af lysine. Enzymet indgår også i omsætning af tryptophan.
Mitochondrial 2-oxoglutarate-dehydrogenase-complex-like protein består af:
DHTKD1, dehydrogenase E1 and transketolase domain containing 1
og så mener man, at enzymkomplekset deler E2 og E3 subunit med OGDC (3).
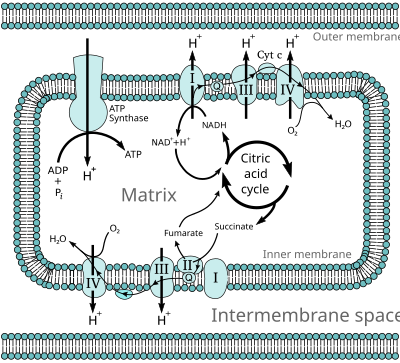
2-ketoacid dehydrogenaserne og deres betydning for TCA cyklus er vist i figur 3 i reference 2:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5961061/figure/F3/
Nu standser vi op et øjeblik og tænker! Hypotesen siger: "Impairment of the E3 subunit...". Hvis hypotesen er sand på dette punkt, kan der forventes en påvirkning af alle enzym komplekser, der anvender E3 subunit DLD. Det vil jeg vende tilbage til. Vi skal først forstå lipoic acid's rolle til bunds.
Dannelse af lipoylated E2 subunit
Når lipoic acid bygges sammen med et protein, f.eks. enzymet subunit E2, kaldes det en lipoylation. Proteinet er blevet lipoylated. Den biokemiske stivej, der sørger for dette er ikke 100% klarlagt, så man møder en del uafklarede spørgsmål i litteraturen.
Figur 1B i reference 2 viser bedste bud på lipoylation af E2 subunit, som man mener, at det foregår hos mennesker.
Først dannes en octanoyl-ACP. ACP kaldes også for
NDUFAB1 (ikke vist på figuren). Og så følger de tre trin beskrevet i figuren, hvor tre enzymer benyttes:
LIPT2, lipoyl (octanoyl) transferase 2
LIAS, lipoic acid synthetase
LIPT1, lipoyltransferase 1
Structures, enzymes, and reaction mechanisms of lipoic acid metabolism (figur 1 fra reference 2). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5961061/figure/F1/
Jeg vil i det følgende gennemgå figuren, men først forklarer jeg dannelsen af octanoyl-ACP.
Dannelse af octanoyl-ACP
Octanoic acid er en organisk syre. En organisk syre er kendetegnet ved at have en -COOH gruppe. Når -OH gruppen fjernes fra en organisk syre, så opstår en acyl, som er et mellemprodukt til en videre biokemisk reaktion. Man benytter bogstaverne -oyl i nanvnet på den opståede acyl-gruppe.Enkelte af de kemiske forbindelser kaldes dog for -yl, som f.eks. formyl og acetyl.
Når octanoic acid får fjernet sin OH gruppe bliver den til en octanoyl. Dette octanoyl mellemprodukt bæres rundt i cellen af et acyl carrier protein (ACP). Det hedder en octanoyl-ACP. Octanoyl og ACP er bundet sammen via et svovl atom (-S-).
LIPT2 - første trin i dannelse af lipoylated E2 subunit: Octanoyl-H-protein dannes
LIPT2 er genet for enzymet lipoyl (octanoyl) transferase 2. Transfer bedtyder overfører. LIPT2 overfører octanoyl fra octanoyl-ACP til et protein H under fraspaltning af ACP. Hermed dannes octanoyl-H protein. (Protein H anvendes også i GCS komplekset). Octanoyl og H protein er bundet sammen med en amid binding (-NH-). Dette er illustreret i første trin i figur 1B i reference 2.
LIAS - andet trin i dannelse af lipoylated E2 subunit: Lipoylated H-protein dannes
LIAS er genet for enzymet lipoic acid synthetase. LIAS sætter to svovl atomer (S) på octanoyl-H proteinet. Hermed dannes lipoylated H protein. Dvs et protein koblet sammen med lipoic acid. LIAS anvender svovl-atom donation fra et (4Fe-4S) cluster og reduktiv spaltning af S-adenosylmethionine (SAM) for at danne 5'deoxyadenosyl-5'radikaler, der kan fjerne hydrogenatomer (H) fra kulstof atom (C) nr 6 og nr 8 og i stedet indsætte svovatomer (S). Dette er illustreret i andet trin i figur 1B fra reference 2.
LIPT1 - tredie trin i dannelse af lipoylated E2 subunit: Lipoylated E2 subunit dannes
LIPT1 er genet for enzymet lipoyltransferase 1. LIPT1 fjerner H protein fra octanoyl og sætter i stedet E2 subunit på. Hermed dannes lipoylated E2 subunit. Dette er illustreret i tredie trin i figur 1B fra reference 2.
Af reference 4 figur 1 fremgår det, at lipoylated E2 subunit kan dannes UDEN først at anvende et H-protein:
Fig 1. Lipoic acid biosynthesis.
mtFAS generates octanoyl-ACP, that enters the lipoic acid biosynthesis pathway. The octanoyl moiety is then transferred from ACP to H or E2 proteins. Subsequently, insertion of two sulfur atoms occurs on the octanoyl moiety to generate lipoylated H or E2 proteins. 2-KGDH, α-ketoglutarate dehydrogenase; 2-OADH, 2-oxoadipate dehydrogenase; ACP, acyl carrier protein; BCKDH, branched-chain ketoacid dehydrogenase; GCS, glycine cleavage system; LA, lipoic acid; LIAS, lipoic acid synthetase; LIPT1, lipoyl(octanoyl) transferase 1; LIPT2, lipoyl(octanoyl) transferase 2; mtFAS, mitochondrial fatty acidynthesis; PDH, pyruvate dehydrogenase.
Dihydrolipoamide
E3 subunit hedder "dihydrolipoamide dehydrogenase. Dihdro betyder to hydrogen (H). Det betyder, at svovlbindingen i lipoic acid er brudt, og der er sat en H på hver S. Amid er en -NH2, der er sat på C=O, dvs en amid er det neutrale derivat af en carboxylsyre. Hvis det ene H fjernes, så har vi en amid-bindingsgruppe -NH-, som kan kobles på en E2 subunit. E3 subunit fjerner to H, dvs den dehydrogenerer dihydrolipoamide. Heraf navnet dihyrolipoamide dehydrogense.
Det skal fremhæves, at artiklen påpeger, at kosttilskud af lipoic acid (LA) ikke hjælper: "
The lack of an independent salvage pathway in humans abrogates the use of LA supplementation as a therapeutic option" (2).
2-keto-acid dehydrogenase komplekser i funktion
Figur 2 i reference 2 viser hvordan de tre subunits fungerer i samspil:
E2 overfører acyl-gruppen til Coenzym A (CoA). Herved dannes en acyl-CoA og den E2 bundne lipoic acid (LA) bliver til dihydrolipoamide (DHLA).
E3: Lipoic acid har under processen i E2 modtaget to hydrogen og er blevet til dihydrolipoamide (DHLA). Der er en sving-arm mellem E2 og E3, der flytter DHLA molekylet fra E2 til E3, I E3 fjernes de to hydrogen atomer og DHLA bliver herved regeneret til LA. Svingarmen kommer tilbage til E2 med LA. E3 benytter FAD og NAD til regenerering af DHLA til LA.
E3 subunit's kapacitet til at regenerere oxideret lipoic acid kontrolleres af NAD+/NADH ratio i mitokondrierne. Når der mangler NAD+, oxideres FADH2 i E3 subunit, og der dannes frie radikaler, som inaktiverer alpha-keto acid komplekserne.
PDC og OGDC kan beskyttes mod oxidativt stress ved glutathionylation. Gluathionylation inaktiverer enzymkomplekset. Glutathione-modifikationen kan igen fjernes med glutathione reductase 2, som kodes af genet
GLRX2. OGDC bliver også beskyttet af thioredoxin reductase 2, som kodes af genet
TXNRD2.
Figur 4 i reference to viser "Regulation of OGDH by reversible glutathionylation":
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5961061/figure/F4/
ME/SEID hypotese sammenholdt med ME forskningen
ME forskning der viser påvirkning af PDC
Nedsat PDC funktion hos ME patienter er allerede sandsynliggjort i Fluge, Mella et al's studie (5).
Det øgede lactat niveau er foreneligt med nedsat PDC funktion. Øget lactat niveau hos ME patienter i forbindelse med motion er for nylig dokumenteret af Lien et al (6).
ME forskning der viser påvirkning af PDHX
PDHX gene promoter var hypomethyleret i peripheral blood mononuclear blood cells (PBMC) fra ME patienter (7).
PDHX genekspression i PBMC ændres ved motion (8). McGregor et al (9) henviser til reference 8 i deres tabel S1, hvor opmærksomheden henledes på, at PDHX er reguleret af histone deacetylaserne
HDAC1 og
HDAC2. Histone regulering er påvirket hos ME patienter i forbindelse med post exertional malaise (PEM) (9).
ME forskning der viser påvirkning af DLAT (E2 subnit i PDC)
Sirtuin 4 (
SIRT4) er et enzym med flere funktioner. SIRT4 kan fungere som E2 subunit lipomidase og klippe lipoamiden af E2 subuniten, og hermed nedregulere PDC aktivitet. Citat fra reference 10:
"We determine that SIRT4 enzymatically hydrolyzes the lipoamide cofactors from the E2 component dihydrolipoyllysine acetyltransferase (DLAT), diminishing PDH activity. We demonstrate SIRT4-mediated regulation of DLAT lipoyl levels and PDH activity in cells and in vivo, in mouse liver."
Figur 1 fra reference 10:
SIRT4 interacts with the pyruvate dehydrogenase complex
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4344121/figure/F1/
Der blev fundet øget mRNA ekspression af SIRT4 i PBMC fra ME patienter (p = 0,013) (5).
Der er blevet påvist spor af DLAT i spinalvæske fra ME patienter, men man ikke fandt DLAT spor hos kontrol personer eller hos Lyme patienter (patienter behandlet for borrelia infektion):
DLAT precursor, number of unique peptides identified in cerebrospinal fluid (table S1 in ref 11):
1) Controls: ingen værdi
2) ME patients: 1
3) Post treatment Lyme patients: ingen værdi
ME forskning der viser/ikke viser(?) påvirkning af DLD (den fælles E3 subnit)
Hvis DLD ikke fungerer kan man forvente, at alle DLD afhængige enzym komplekser er påvirkede. En genetisk mutation kan være dødelig (12): "
E3-deficiency is a rare autosomal recessive genetic disorder frequently presenting with a neonatal onset and premature death."
Man må formode, at en mindre påvirkning af DLD vil give en mindre grad af alvorlighed. Spørgsmålet er således. Er alle DLD afhængige enzymer påvirkede hos ME patienter? Vi kender ikke svaret, men vi må holde øje med, hvad forskningen viser os.
En hæmning af OGDH vil give forhøjet niveau af alpha-ketoglutarate og 2-hydroxyglutarate, jvf figur 3 i reference 2. Et studie har vist forhøjet plasma niveau af alpha-ketoglutarate hos ME patienter (p = 0,003) (13). Gen ekspression af enzymet D-2-hydroxyglutarate dehydrogenase (D2HGDH) var opreguleret i fuldblod fra teen-age CFS patienter (p = 0,0555) (14). Genet D2HGDH var hypomethyleret (body) i PBMC fra ME patienter (15).
ME patienter anvender forgrenede aminosyrer som brændstof (5), så det må formodes at BCKDC fungerer.
Subunit E2 fra BCKDC hedder dihydrolipoamide branched chain transacylase E2 (DBT), og kaldes også for dihydrolipoyllysine-residue (2-methylpropanoyl) transferase. Plasma niveauet af S-(2-methylpropanoyl)-dihydrolipoamide var formindsket i plasma fra ME patienter (16). Hvad betyder dette fund?
Hvis glycine cleavage system (GCS) ikke fungerer, kommer der en ophobning af glycine. Glycine niveauet er normalt hos ME patienter. I reference 5 har ME pateinter et lidt højere serum niveau af glycine, men det er ikke statistisk signifikant (p = 0,082).
Gen promotor for AMT (som er den del af GCS) var hypomethyleret i PBMC fra ME patienter (7). Hvorfor?
ME forskning der viser påvirkning af mitokondrie fedtsyrer syntese - octanoic acid
Som det fremgår af reference 2, så starter produktionen af E2 subunit helt fra bunden af med en fedtsyre med 8 kulstofatmer, octanoic acid, (C8:0).
To uafhængig studier har påvist formindsket plasma niveau af 2-octenoylcarnitine (C8:1) hos ME patienter. Dvs carnitninen er sat sammen med en otte-kædet fedtsyre med en dobbeltbinding (17, 18). Naviaux et al (17) kommenterer resultatet således:
"Octenoic acid is produced as an intermediate of mitochondrial fatty acid and lipoic acid synthesis. It is the substrate for mitochondrial enoyl thioester reductase (ETR), a family of enzymes that requires NADPH to reduce the double bond to octanoic acid, which is then used for lipoic acid synthesis. Decreased levels of this C8:1 acylcarnitine are consistent with decreased mitochondrial fatty acid synthesis, increased oxidation, increased renal secretion, or a combination of the three."
ME forskning der viser påvirkning af mitokondrie fedtsyrer syntese - ACSM1
Der er diskussion om acyl-CoA synthetase medium chain family member 1,
ACSM1, påvirker lipoic acid syntesen (2). I reference 2 står der:
"There has been a report identifying a mammalian lipoic acid-activating enzyme that could activate exogenous lipoic acid (36); however, this function was ultimately attributed to the mitochondrial medium-chain acyl-CoA synthetase (ACSM1) (37, 38). This enzyme can utilize both the (R)- and (S)-enantiomers of LA and primarily uses GTP to activate the natural (R)-lipoic acid, but so far there has been no substantial evidence to support that this enzyme functions in LA metabolism in vivo (36). "
Om det er relevant eller ej skal det nævnes, at genet ACSM1 (TSS200) er påvist hypomethyleret i PBMC fra ME patienter i to uafhængige studier (7, 15). Ydermere er forskellig methylering af ACSM1 relateret til ME patient subtypes (19).
ME forskning der viser påvirkning af mitokondrie fedtsyrer syntese - ACACA og ACSF3
Malonyl-CoA er nødvendig for fedtsyre syntese i mitokondrierne og hermed også nødvendig for dannelse af lipoic acid. Acetyl-CoA carboxylase alpha (ACACA) katalyserer omdannelse af acetyl-CoA til malonyl-CoA. Acyl-CoA synthetase family member 3 (ACSF3) kan også danne malonyl-CoA. En mitokondriel isoform af ACACA og ACSF3 sørger i fællesskab for, at der er nok malonyl-CoA til at danne lipoic acid (20).
DNA methylering af ACACA og ACSF3 i PBMC fra ME patienter:
ACACA hypermethylering: 5'UTR;body og body (15), og body (21)
ACACA hypomethylering: to steder i 5'UTR, og to steder i en genic location, der ikke er angivet (7)
ACSF3 hypermethylering: tre steder body (15)
ACSF3 hypomethylering: tre steder 5'UTR og TSS100 (7), og body;5'UTR (15)
Forskellig methylering af ACSF3 (body) var relateret til ME patient subtypes (19).
ME forskning der viser påvirkning af LIPT1
Nogle mutationer i LIPT1 forårsager manglende aktivitet af PDC og OGDC, mens GCS stadig fungerer (22, 23). Der er rapporteret om dødelig lactis acidose i forbindelse med LIPT1 mutationer (24).
LIPT1 var hypermethyleret med en foldchange = 5,9 og p = 0,03 i CD4+ T celler fra ME patienter (25).
ME forskning der viser påvirkning af thioredoxin reductase og glutaredoxin
TXNDR1 var hypomethyleret med en negativ foldchange =
-3,6 og p = 0,000165 i CD4+ T celler fra ME patietner (25).
GLRX2 var hypermetyleret (TSS1500) i PBMC fra ME patienter i to studier (15, 21).
Gen ekspression af
GLRX var opreguleret i fuldblod fra teen-age CFS patienter (p = 0,0005), hvilket var den laveste p-værdi i studiet (14).
Mere viden:
Thioredoxin and glutaredoxin-mediated redox regulation of ribonucleotide reductase
Videre inspiration
Tak til Victoria Bohne, Oeyvind Bohne og de personer, der donerede til illustration af deres artikel om ME/SEID hypotesen.
Vi skal helt sikkert et spadestik dybere ned i hvad der foregår i pyruvat dehydrogenase komplekset. Videre læsning må være:
Structural and Functional Analyses of the Human PDH Complex Suggest a “Division-of-Labor” Mechanism by Local E1 and E3 Clusters
7) Trivedi et al: Identification of ME/CFS - associated DNA methylation patterns. Plos One 2018, 13, 7
8) Whistler et al: Exercise responsive genes measured in peripheral blood of women with chronic fatigue syndrome and matched control subjects
2005 Mar 24;5(1):5.
9) Mcgregor et al: Post-Exertional Malaise Is Associated with Hypermetabolism, Hypoacetylation and Purine Metabolism Deregulation in ME/CFS Cases
2019 Jul 4;9(3). pii: E70. doi: 10.3390/diagnostics9030070.
10) Mathias et al: Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity.
2014 Dec 18;159(7):1615-25. doi: 10.1016/j.cell.2014.11.046.
11) Schutzer et al: Distinct Cerebrospinal Fluid Proteomes Differentiate Post- Treatment Lyme Disease from Chronic Fatigue Syndrome. PLOS One February 2011, volume 6, Issue
13) Germain et al: Prospective Biomarkers from Plasma Metabolomics of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Implicate Redox Imbalance in Disease Symptomatology.
2018 Dec 6;8(4). pii: E90. doi: 10.3390/metabo8040090.
14) Nguyen et al: Whole blood gene expression in adolescent CFS: an exploratory crosssectional study suggesting altered B cell differentiation and survival. J Transl Med. 2017,15,102.
17) Naviaux RK, Naviaux JC, Li K, Bright AT, Alaynick WA, Wang L, Baxter A, Nathan N et al (2016) Metabolic features of chronic fatigue syndrome. Proc Natl Acad Sci U S A 113:E5472–E5480.
18) Reuter and Evans: Long-chain acylcarnitine deficiency in patients with CFS. Potential involvement of altered carnitine palmitoyltransferase-I-activity. J. Int. Med. 2011, 270.